
Chem Ions Scientific Tutor
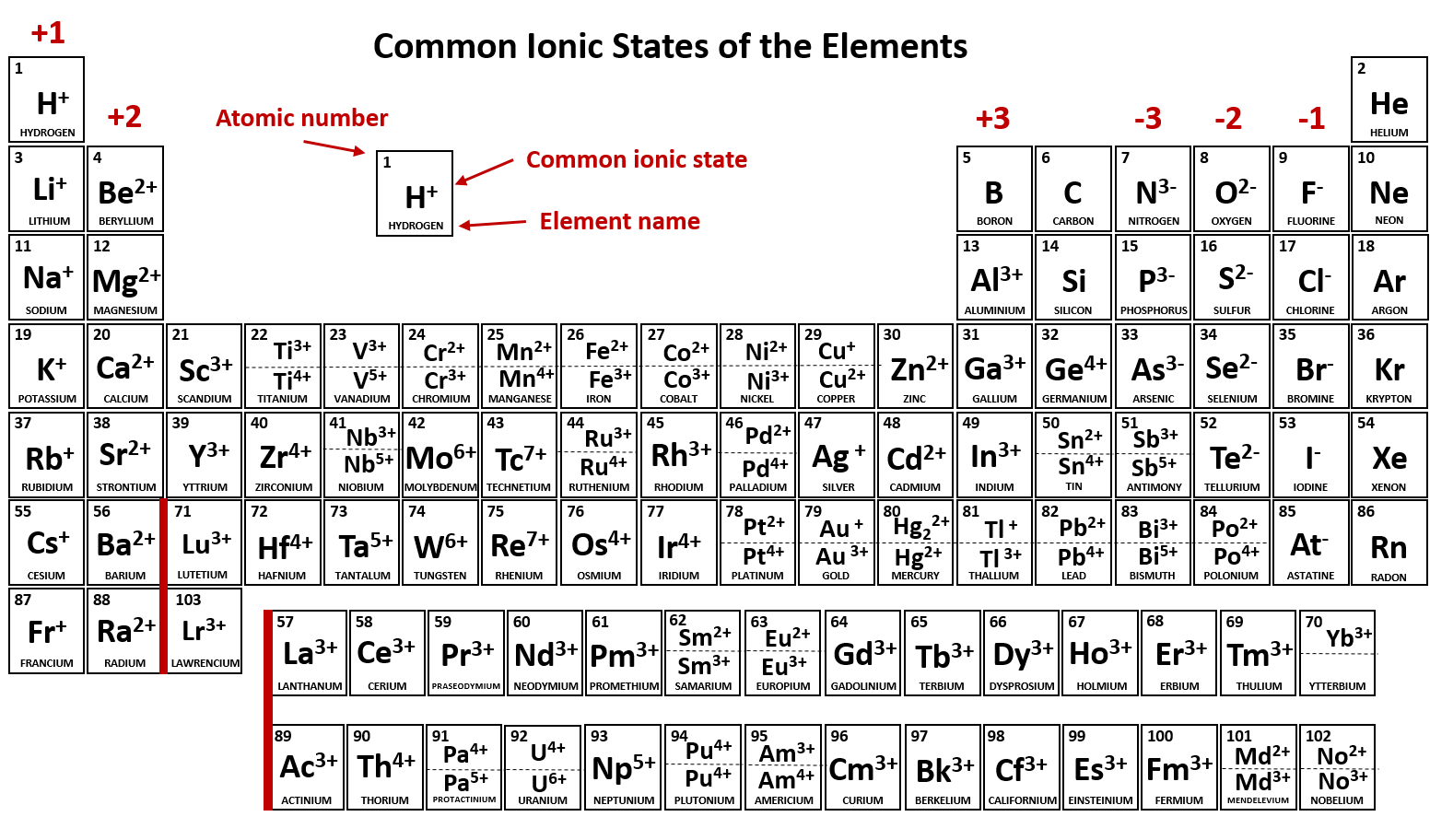
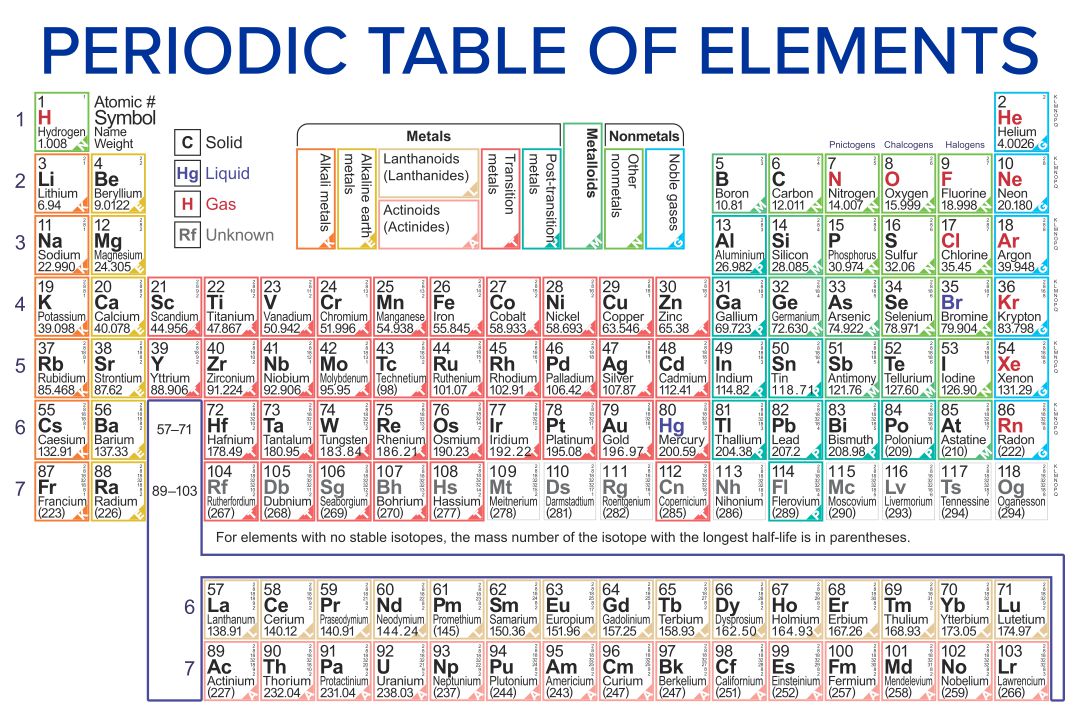
Note the usefulness of the periodic table in predicting likely ion formation and charge (Figure \(\PageIndex{2}\)). Moving from the far left to the right on the periodic table, main-group elements tend to form cations with a charge equal to the group number. That is, group 1 elements form 1+ ions; group 2 elements form 2+ ions, and so on.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Periodic Table with Ions . 3.3 Ionic Bonding. Most of the rocks and minerals that make up the Earth's crust are composed of positive and negative ions held together by ionic bonding. An ionic compound is an electrically neutral compound consisting of positive and negative ions.

Ionic Periodic Table Free Download To Print Online
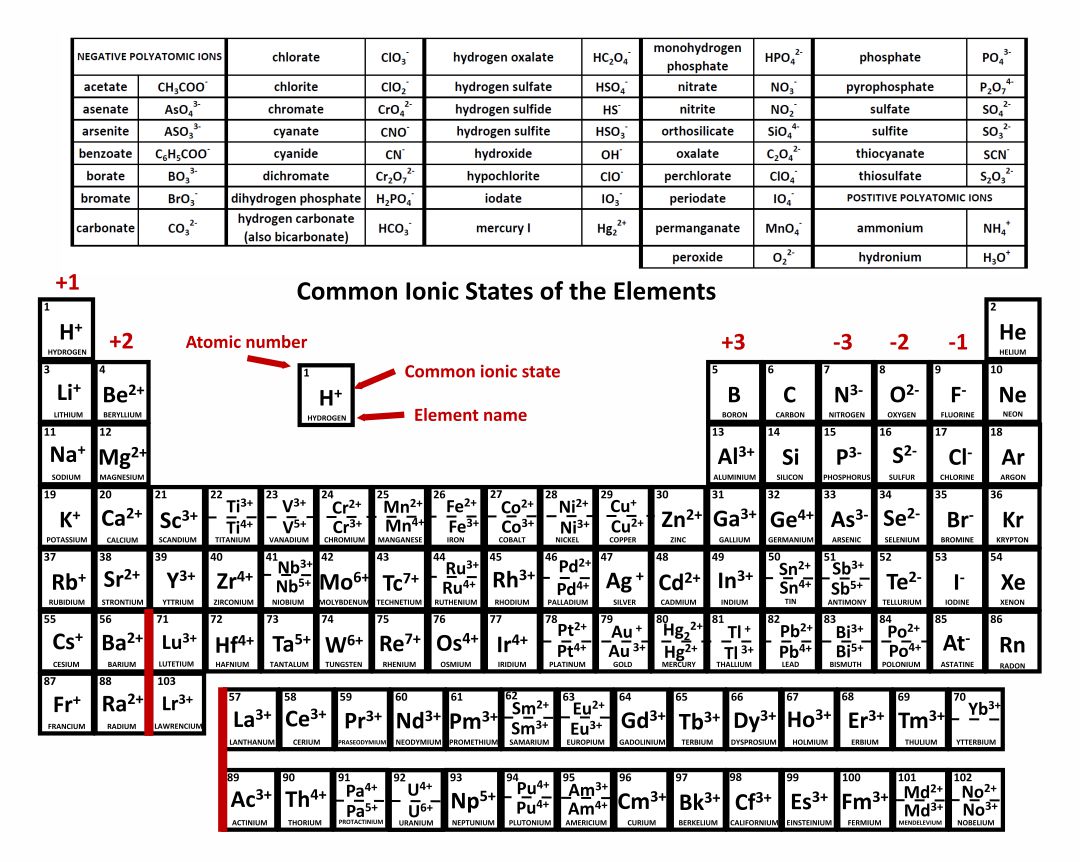
h+. hydrogen. 1. li + lithium. 3. na + sodium. 11. k + potassium. 19. rb + rubidium. 37. cs + cesium. 55. fr + francium. 87. be. 2+ beryllium. 4. mg. 2+ magnesium. 12.
10 Best Printable Periodic Table Of Ions PDF for Free at Printablee
Ions are atoms that have a positive or negative charge because they have unequal numbers of protons and electrons. If atoms lose electrons, they become positive ions, or cations. If atoms gain electrons, they become negative ions, or anions. Consider the example of fluorine (see figure below). A fluorine atom has nine protons and nine electrons.
Periodic Table Ions List Periodic Table Timeline
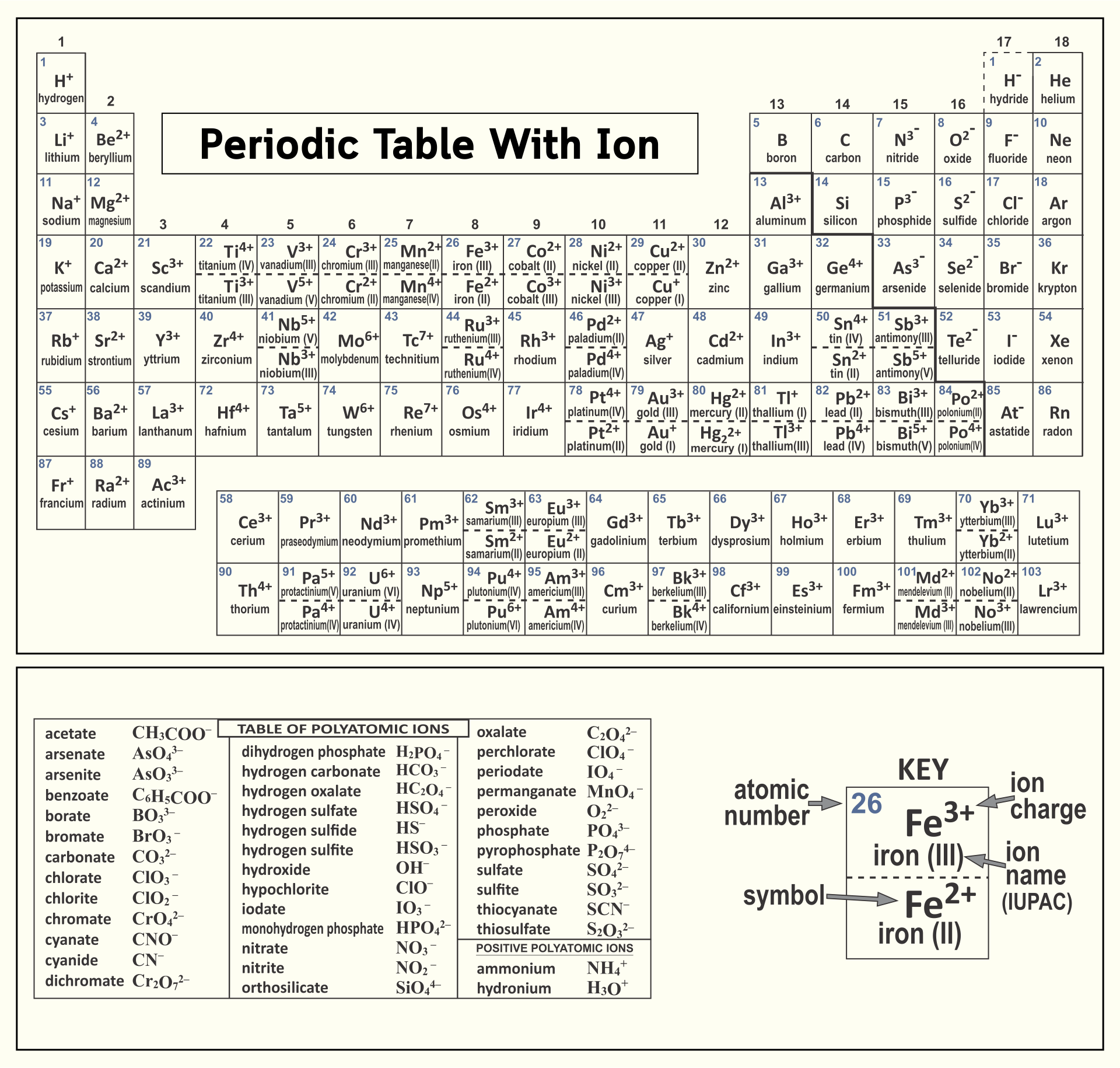
"An ion is a small electrically charged particle. Ions are single charged atoms (simple ions) or small charged "molecules" (polyatomic ions)." Simple ions include Na +, Ca 2+, and Cl -. Polyatomic ions include (NH 4) +, (CO 3) 2-, and OH -. Unlike protons and neutrons, electrons can be easily removed and added to an atom.
10 Best Printable Periodic Table Of Ions PDF for Free at Printablee
Atoms are electrically neutral because the number of protons, which carry a 1+ charge, in the nucleus of an atom is equal to the number of electrons, which carry a 1- charge, in the atom. The result is that the total positive charge of the protons cancels out the total negative charge of the electrons so that the net charge of the atom is zero.
10 Best Printable Periodic Table Of Ions PDF for Free at Printablee
acetate PERIODIC TABLE OF IONS arsenate arsenite benzoate borate bromate carbonate chlorate chlorite chromate cyanate cyanide dichromate CH3COO- AsO4 3- AsO3 3- C6H5COO - BO3 3- BrO3 - CO3 2- ClO3 - ClO2 - CrO4 2- CNO- CN- Cr2O7 2- oxalate perchlorate periodate permanganate peroxide phosphate pyrophosphate sulfate.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Learning Objectives By the end of this section, you will be able to: Define ions Identify the charges of the ions formed by main group elements on the periodic table Ions As a recap from Chapter 3, during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (Figure 6.1a).

Printable Periodic Table With Polyatomic Ions Periodic Table Timeline
High school chemistry 9 units · 55 skills. Unit 1 Atoms, elements, and the periodic table. Unit 2 Chemical bonding. Unit 3 Chemical reactions. Unit 4 Stoichiometry and the mole. Unit 5 States of matter. Unit 6 Thermochemistry. Unit 7 Solutions, acids, and bases. Unit 8 Reaction rates and equilibrium.

Labeled Periodic Table With Ionic Charges Periodic Table Timeline
The most-requested printable periodic table lists element charges, to predict compounds and chemical reactions. Now, you can use periodic table trends to predict the most common element charges.

Ions
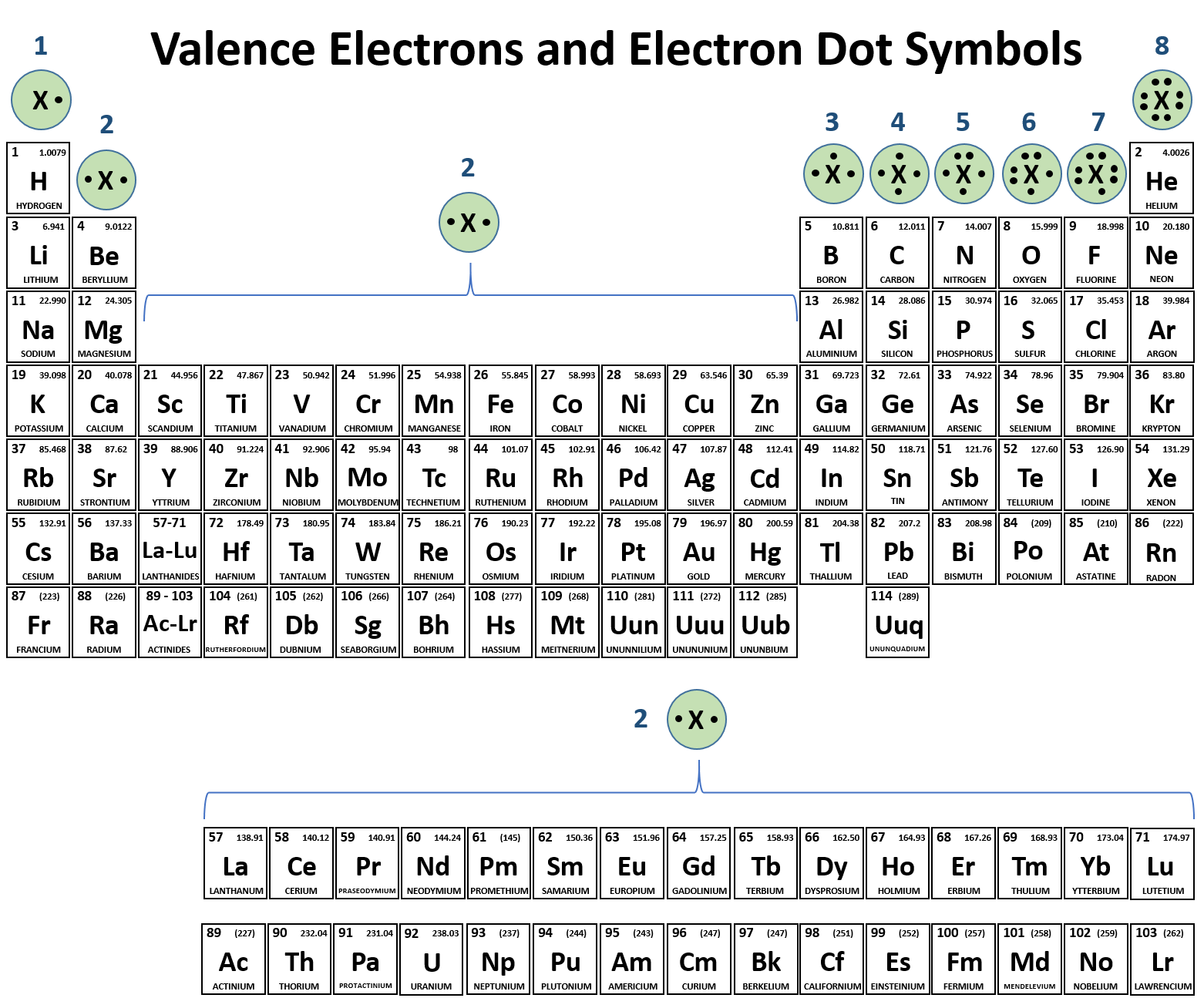
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.
:max_bytes(150000):strip_icc()/PeriodicTableCharge-WBG-56a12db23df78cf772682c37.png)
Periodic Table With Common Ionic Charges
Periodic Table of Elements TABLE LIST W/PROPERTIES GAME Display Property/Trend 17 Cl Chlorine halogen Plot Atomic Mass 1 H Hydrogen nonmetal 2 He Helium noble gas 3 Li Lithium alkali metal 4 Be Beryllium alkaline earth metal 5 B Boron metalloid 6 C Carbon nonmetal 7 N Nitrogen nonmetal 8

periodic table of ions printable Periodic Chart of Ions PDF Science ) Pinterest
Common charges of ions formed by elements in different groups of the periodic table. Note: Hydrogen is somewhat unusual in that it readily forms both cations and anions. Most elements form only one or the other. Naming cations How do we name main group cations?

Naming Simple Ionic Compounds Pathways to Chemistry
When atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic table. Atoms of group 17 gain one electron and form anions with a 1− charge; atoms of group 16 gain two electrons and form ions with a 2− charge, and so on.

Periodic table with charges of ions bezygps
An atom becomes an ion, or a charged atom, because of the gain or loss of electrons. Ionic size changes depending on the charge of the ion. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. Negative ions are larger than their source atom because of the gain.
10 Best Printable Periodic Table Of Ions PDF for Free at Printablee
Table of Polyatomic Ions. acetate CH 3COO- dichromate Cr 2O 2-. 7 dihydrogen phosphate H 2PO -. 4. ammonium NH4 + cyanide CN- silicate 2- SiO3 benzoate C6H5COO- hydroxide OH- sulphate SO4 2-. borate BO3 3- iodate - IO3 sulphite 2- SO3 carbonate 2- CO3 nitrate - NO3 hydrogen sulphide HS-.